- For U.S. Healthcare Professionals Only
Safety profile demonstrated in adult patients in a Phase 3 pivotal trial
Lower rate of treatment discontinuation due to AEs with LIVTENCITY vs IAT1
Discontinuation rates:
(n=31/234)2
of patients receiving LIVTENCITY
vs
(n=37/116)2
of patients receiving IAT
LIVTENCITY
Dysgeusia, diarrhea, nausea, and recurrence of underlying disease (each reported at 1%)
Most common reasons for treatment discontinuation
IAT
Neutropenia
IAT
Acute kidney injury
AE=adverse event.
Adverse events (all grades) reported in >10% of adult patients in the LIVTENCITY group1
The mean treatment durations (SD) for LIVTENCITY and IAT were 48.6 (± 13.82) and 31.2 (± 16.91) days, respectively.1
| ADVERSE EVENT | LIVTENCITY | IAT* |
|---|---|---|
Taste disturbance† | 46 | 4 |
Nausea | 21 | 22 |
Diarrhea | 19 | 21 |
Vomiting | 14 | 16 |
Fatigue | 12 | 9 |
*IAT (investigator-assigned treatment) included monotherapy or dual therapy with ganciclovir, valganciclovir, foscarnet, or cidofovir as dosed by the investigator.
†Taste disturbance includes the following reported preferred terms: ageusia, dysgeusia, hypogeusia and taste disorder.
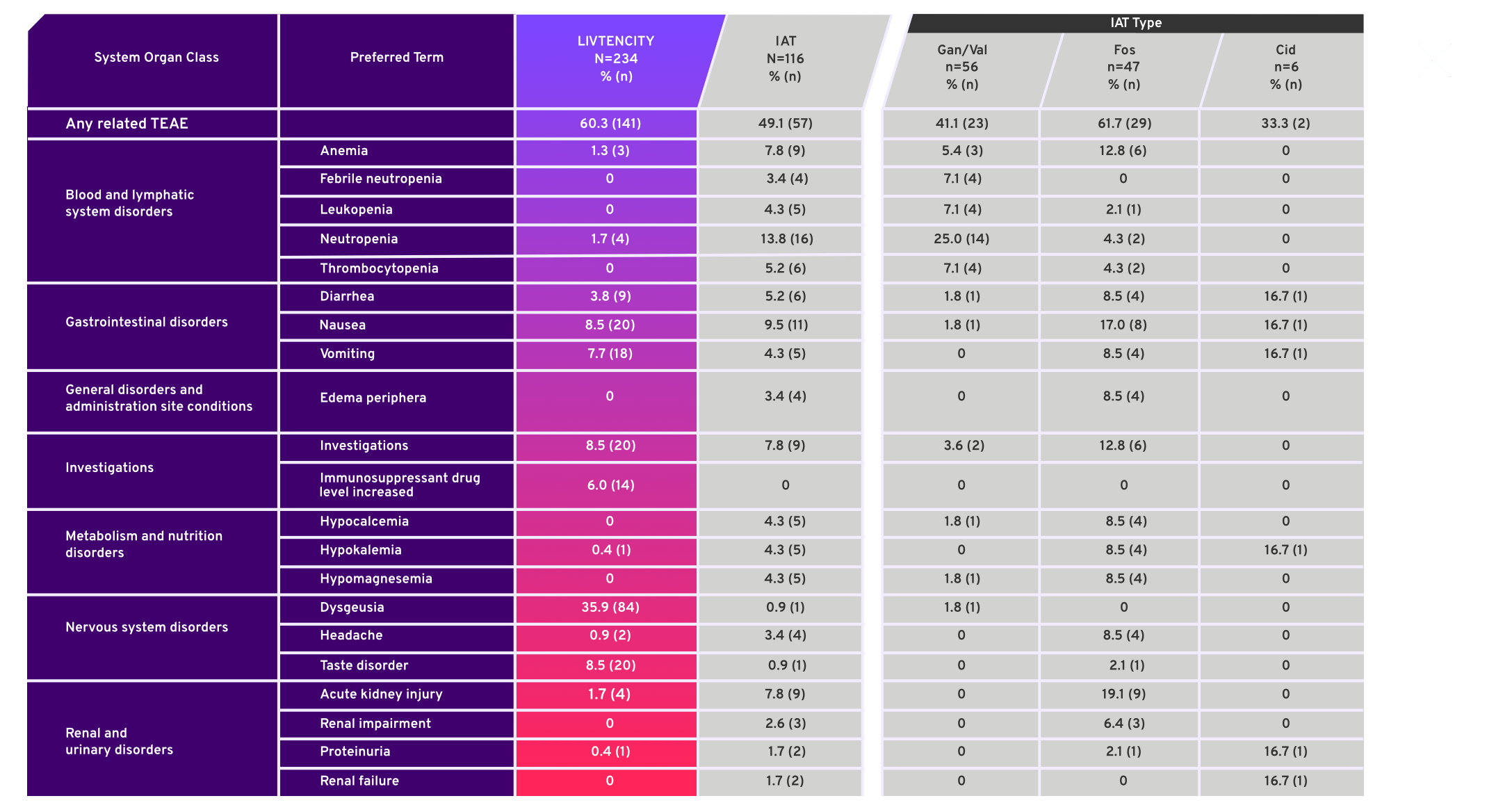
Treatment emergent adverse events (TEAE) in ≥5% of patients in either treatment group or for individual IAT, considered related to study-assigned treatment by the investigator4*
- SOLSTICE, a Phase 3, multicenter, randomized, open-label, active-controlled trial, compared LIVTENCITY to Investigator-Assigned Treatment (IAT)
- Study was not designed to evaluate safety differences between individual IAT types, therefore comparisons of safety profiles cannot be made
- Safety data were analyzed descriptively in all patients who received a dose of study drug (Safety Population)3
Taste disturbance rarely led to treatment discontinuation and usually resolved while on or post-treatment1,3
Taste disturbance was the most common AE reported in patients receiving LIVTENCITY | 46% (n=108/234)2 experienced taste disturbance | In some patients, taste disturbance resolved during treatment | 37% (n=44/119)2 had resolution while on treatment | Taste disturbance rarely led to treatment discontinuation | 1% (n=2/234)2 discontinued due to taste disturbance |
|---|
- For patients with ongoing taste disturbance after drug discontinuation, resolution occured in 89%1
- In patients with resolution of symptoms after drug discontinuation, the median duration of symptoms off treatment was 6 days (range 2 to 85 days)1
Taste disturbance included the terms: ageusia, dysgeusia, hypogeusia, and taste disorder.
Selected laboratory abnormalities1
Reported by central laboratory with samples collected every 2 weeks*
| Laboratory parameter | LIVTENCITY | IAT |
|---|---|---|
Neutrophils (cells/μL) | 2% (4) | 3% (4) |
Hemoglobin (g/dL) | 1% (3) | 1% (1) |
Platelets (cells/μL) | 5% (11) | 5% (6) |
Creatinine (mg/dL) | 7% (16) | 10% (12) |
*As specified by detailed protocol design per Data on File.
Explore what LIVTENCITY dosing could mean for your patients.

You are being directed to another website.